Biosafety
Information about infectious agents, genetic modifications, containment measures, risk assessments, permits and legal requirements
Biological risks
Biologiska risker
Biological risks can be infectious substances, genetically modified microorganisms (GMM), blood & tissue, research animals, invasive species, or waste thereof, which can have a detrimental impact on human health or the environment at large.

Examples of what can constitute a biorisks: Toxins, Prions, Viruses, Bacteria, Fungi, Parasites, Cell cultures, Blood and tissue, Research animals, Plants, and Laboratory waste
Legislation, containment measures, and risk assessment
When working with infectious agents and genetically modified microorganisms (GMM) there legal requirements in the provisions ”smittrisk” and "Innesluten användning av genetiskt modifierade mikroorganismer" (especially page 10-15), which specify the containment measures required for work with infectious agents and GMM in different risk classes. For example, when gloves and lab coat is required and when the sign ”Biological Hazard” must be placed on the door to the lab.
Warningsign for Biological Hazard Word, 358 kB.
Apart from these general safety instructions, it is important that all those who participate in the work preform a risk assessment so that all the individual risks which may occur during the project can be identified, and safety measures implemented. The risk assessment should be kept in the laboratory, readily available to everyone, including government agencies during inspections. Some government agencies include a risk assessment form in the report form, if not here is a general risk assessment form for biorisks.
General risk assessment form Word, 722 kB.
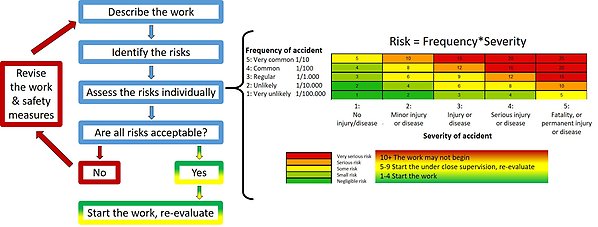
Risk assessment flowchart. Start with a cursory description of the work, identify individual risks, assess, if needed modify the safety features to lower the risks.
A part of the risk assessment is to write instructions for handling an infections spill. Download these general instructions, adapt them to the risks in your laboratory, print them, and keep them in the groups’ spill-kit together with personal protective equipment and warning sign for biological spill.
Genneral instructions for a spill-kit Word, 585 kB.
Information about general risk management in laboratory work
Human samples, COVID-19 patient samples, and propagation of SARS-CoV-2
According the provisions governing work with infectious agents (Smittrisk AFS 2018:4), human samples should in general be handled at containment level-2. For human samples with higher risk, e.g. COVID-19 patient samples, it could be prudent with additional containment measures. Propagation of SARS-CoV-2 samples should be handled at containment level-3. Se information from The Public Health Agency of Sweden for details.
Risk of infection & Vaccination
There is always a risk of infection when working with tissue, bodily fluids, or pure infectious agents, especially so during aerosol formation, and work with needles and scalpels. Vaccination can be a good complement to the regular protective measures taken. It is however important to remember that vaccination does not have 100% efficacy.
The general guideline is that all those how work with human tissue or bodily fluids should be vaccinated against Hepatitis A & B. The need for vaccination is however always determined by the employee and closest supervisor through a risk assessment.
The following aspects should be included in the risk assessment:
- The infectious substance
- The type of work
- The health status of the employee
- The efficacy of the vaccine
- The side effects of the vaccine
If the risk assessment indicates that vaccination is suitable, then send the risk assessment to the prefect/equivalent, how in turn books vaccination according to the institutions routines.
The employer pays for the vaccination, not the employee.
In case of an accident, it is important to have a written routine, which dictates what to do in different situations; e.g. how to handle contact with blood or infectious agents. If you suspect a laboratory related infection, contact your supervisor and if needed the hospitals infection ward 018-611 56 08. Furthermore, the employer is required to document when exposure to infectious substances in risk class 3 or 4 has been ascertained in connection to incidents, accidents or other undesirable events. See infection risk regulation AFS2018:4, § 11 for details.
Information on what to do in case of contact with blood Pdf, 5 MB.
Reporting/Permission for working with infectious substances and genetically modified microorganisms (GMM)
Work with human infectious substances and genetically modified microorganism is primarily regulated by Arbetsmiljöverket (Swedish Work Environment Authority), and should be reported/permit applied for before the work starts. Work with other Genetically Modified Organisms (ex. plants and animals) is regulated by other agencies.

Overview of which type of work requires reporting or a permit from Arbetsmiljöverket. For wild type work with risk class 2-4 must be reported. For work with genetically modified microorganisms, risk class 1-2 must be reported, whereas risk class 3-4 must require a permit.
Link list - Provisions and information from the Genetic Enginering Board
Consult the biosafety coordinator with a copy of the report and risk assessment prior to sending it to Arbetsmiljöverket.
Send minor updates and changes in current GMM reports via the prefect to the biosafety coordinator during April each year. What constitute minor updates and changes is defined at page 28-31 in AFS 2011:2
Import and usage of biological samples for research
Permits for import and usage of non-human pathogenic biological samples* for research and education are often handled by Jordbruksverket (Swedish Board of Agriculture). In order to reduce administration and costs for individual research groups, Uppsala University has an umbrella permit for import and usage of some biological samples* for research and education from Jordbruksverket.
This means that individual research groups do not have to apply for these permits, but simply fill out this form and e-mail it to the biosafety coordinator before each import. The research groups are then sent an internal permit and the permit number that suppliers sometimes demand before sending certain orders. The university then sends a central report to Jordbruksverket each semester.
*
- Animal by-products.
- Dead lower animalia which are not endangered.
- N.B. Human-pathogens should not be registered here as import permit for these are included in the report/permit to Arbetsmiljöverket (Swedish Work Environment Authority)
Biorisk committee
The Uppsala biorisk committee establishes general guidelines for work with biological risks. The chairman of the biorisk committee is Professor Staffan Svärd, and the biosafety coordinator acts as secretary and contact-person to whom researchers can come with all questions concerning biorisk, e.g. handling of infectious substances, Genetically Modified Microorganisms, Invasive species, and contact with government agencies, etc.
Members of the Biorisk Committee:
- Magnus Essand, Professor at the Department of Immunology, Genetics and Pathology
- Henrik Gradstedt (secretary, contact-person), Biosafety coordinator at the Buildings Division
- Joakim Holmdahl, Centrumchef CFVUU
- Peter Lindblad, Professor at the Department of Chemistry - Ångström Laboratory
- Anna Maria Näslund, Working environment engineer at the Buildings Division
- Stefan Schwartz, Professor at the Department of Medical Biochemistry and Microbiology
- Mikael Sellin, Docent at the Department of Medical Biochemistry and Microbiology
- Staffan Svärd (Chairman), Professor at the Department of Cell and Molecular Biology
- Irene Söderhäll, Professor at the Department of Organismal Biology
Courses for working with infectious substances and GMM

For those who do not have the experience or are not acquainted with the Swedish laws and regulations governing work with infectious substances or GMM, the course “Working with Biorisks: Infectious Substances and Genetically Modified Microorganisms” is held several times each semester, 09:00-16:45 at BMC C6:109a
The course is free of charge for those who work at Uppsala University (2.000:- for external participants), and is primarily aimed at bachelor/master students, Ph.D. students, post-docs, laboratory assistants, and new group leaders. During the course we discuss the organization of Uppsala University, Swedish laws and ordinances, work with infectious substances, waste management, incidents and accidents, practical risk assessments, containment and clean-up of a simulated biological spill, how to handle sharps, and biosecurity. The course is held in English, and comes with complementary “fika” and lunch.
For Uppsala University Ph.D. students the course can often yield ”Ph.D. points”, ask your course administrator if this is the case at your institution. The course is not aimed at students in basic education as it does not yield credits ”högskolepoäng”
Sign-up closes the day before the course date, or when fully booked.
If this course date is under-booked (minimum 3 participants, maximum 7) it will be cancelled and you will be offered another date.
If none of the available dates work then it is sometimes possible for you to gather 3-7 participants and book a separate course date by mailing henrik.gradstedt@uu.se
Working with Biorisks: Infectious Substances and Genetically Modified Microorganisms. 09:00-16:45 Wednesday 2024/01/17 FULLY BOOKED
Working with Biorisks: Infectious Substances and Genetically Modified Microorganisms. 09:00-16:45 Friday 2024/02/16
Working with Biorisks: Infectious Substances and Genetically Modified Microorganisms. 09:00-16:45 Friday 2024/03/15
Working with Biorisks: Infectious Substances and Genetically Modified Microorganisms. 09:00-16:45 Wednesday 2024/05/22 Fully booked
